The Wise son:
In clinical trials, it is not always about demonstrating that the new product (or test, or treatment) is superior. Sometimes it is necessary to demonstrate that the product is as good as a reference product, but has some other qualities, such as: fewer side effects, an easier administration or it is more cost-effective, safer, or even may be suited for a wider range of indications.
So, tell me, how to argue the non-inferiority (NI) case? What results are needed to convince the regulatory authorities to approve the new product, and claim that the new product is not inferior to a product that is already on the market?
The Simple son:
We need to select the reference product and design a non-inferiority trial which will randomly expose patients to either the new treatment or control products. Then, we need to decide on a NI margin. This margin is defined as the largest clinically acceptable difference between our new product and the reference device.
Look at this figure:
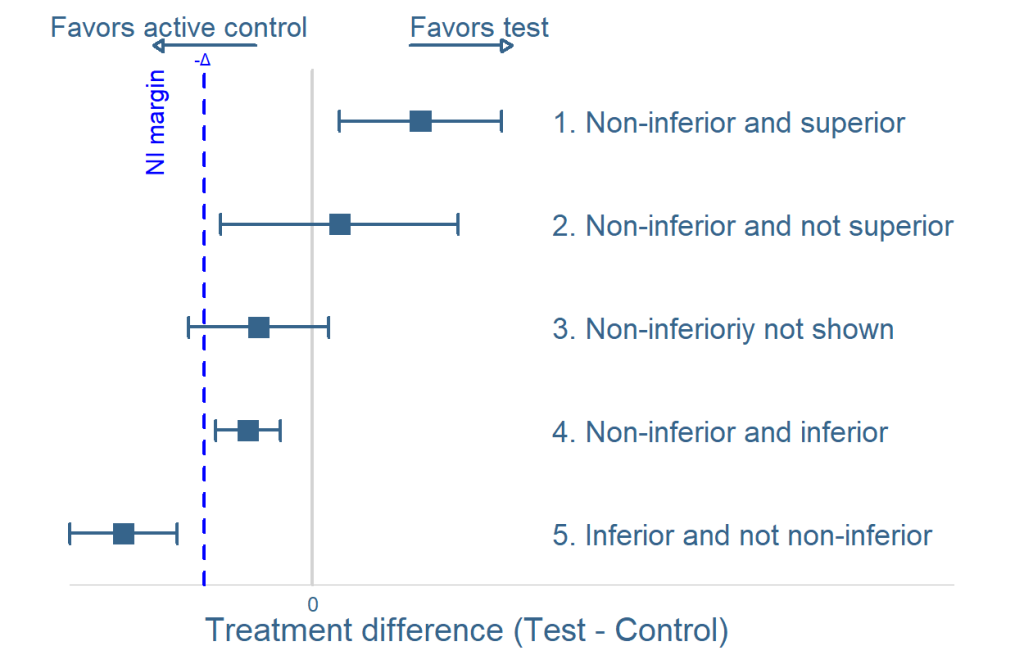
After the study has been completed and the data is evaluated, there are 5 possible outcomes, as demonstrated in the figure: In outcomes 3,4 and 5, inferiority is demonstrated because either the lower 95% confidence interval (CI) limit is below the NI margin or the average is below 0. NI is demonstrated for outcomes where the lower confidence limit is above the margin (1 & 2 in the figure). Should the lower limit exceed zero, superiority of the test device may also be argued.
The Wicked son:
It is so easy to show NI, you just pick a poor reference and a very wide margin, and there you go.
The Simple son:
Well, Wicked, this won’t be easily approved by the regulatory authorities. To assure a successful NI trial, selection of an appropriate reference product and rigorous study execution is required. The analysis should be conducted with both the intent to treat (ITT) and the Per Protocol (PP) datasets, non-inferiority should be demonstrated on both datasets. And yes, the non-inferiority margin should be established in advance with clinicians and regulators.
Furthermore, both the FDA and the European Medicines Agency have published guidelines describing in detail all the considerations for setting NI clinical trials.
He who couldn’t ask:
I need you to design this kind of study for me! I have made a new way of separating egg whites from yolks, using just a tea bag and a juice squeezer. I can assure you, it is not worse than the usual manual separating method! It is just more fun my way!
The Wise son:
Please, get serious here, and tell me, how do I set the required sample size for such NI study?
The Simple son:
I have started to work on some simulations, perhaps on our next post I’ll show you.

