OUR SERVICES
We offer an integrated suite of data analysis services to support the development and marketing of medical products.
Our strong clinical data pipeline is the foundation for effectively addressing all clinical research activities from planning to understanding and insight.
Study Design
Using a shared language with researchers from a range of disciplines we will translate the biomedical problem of interest into a research question. We will propose the appropriate study design while optimizing the execution factors: budget, regulatory and statistical efficiency.
Sample Size Determination
We will help you decide what sample size you need based on study phase and design, effect size, variance and other research area specific considerations. Next, we will provide a “sample-size considerations” document. The process of determining sample size is described in this article. Computational details for multivariate sample size determination are discussed in this article.
Random assignment
IntegriStat offers a web application for clinical study random assignment. The service streamlines the clinical study recruitment method, and is particularly attractive for multi-center and geographically distributed trials.
Features:
· Stratification by key factors (such as Gender, Age, Severity)
· e-mail notification
· Intermediate and final reports
· Code revealing for data-analysis
· Controlled premature un-blinding in case of safety requirement
· Site and user management
· Complies with regulatory requirements
Protocol Write-Up
IntegriStat will provide the statistical methods section for your protocol. Our medical writers can help formulate the clinical protocol and write it up in accordance with good clinical practice (GCP).
Electronic Data Capture (EDC)
IntegriStat provides clinical data management using Electronic Data Capture & Clinical Data Management with our top quality eCRFs. The EDC meets high regulatory requirements including HIPPA, CFR - Title 21 PART-11, GDPR and CDISC. We address additional regulatory issues according to our clients request. IntegriStat has ample experience in constructing EDC to support the trial lifespan from design to data analyses and archival:
• CRF writeup using our in-house CRF library for uniform forms such as selection criteria, medical history, questionnaires, adverse events and change in medication. In addition, we can help you design specific forms adapted to your research.
• EDC buildup to reflect your visits schedule. From intake visit through follow-up to end of study visit. Each visit is represented by an event on the EDC. Unplanned and repeated visits are supported.
• The EDC supports multicenter studies. Each medical center may be customized to local medical practices.
• Users are granted access according to their role in the study: clinical coordinator, study director or data monitor.
• Integristat will train the users to familiarize them with the study EDC as part of the study initiation.
• Integristat provides ongoing support for all EDC activities through our help desk. Should a study require changes after initiation, IntegriStat’s EDC can accommodate CRF version changes.
• IntegriStat will extract the EDC data and supply the sponsor with interim and final reports.
• At the end of the study IntegriStat conducts a data lock to assure data authenticity and electronic data archival.
• All data management activities are conducted according to IntegriStat's in-house Standard Operating Procedures (SOPs).
Statistical Analysis
We do both basic statistical analysis (t-tests, ANOVA, linear and logistic regression, confidence intervals) and advanced modeling (e.g. ROC analysis, survival analysis). We also develop novel procedures when appropriate.
Clinical Trial Report
The clinical study report will be written based on the client’s protocol and the statistical analysis plan. IntegriStat reports satisfy regulatory standards (ICH Efficacy Guideline E3: Structure and Content of Clinical Study Reports). You may request to generate the Clinical Trial Report with the formats indicated by your regulatory affairs requirements.
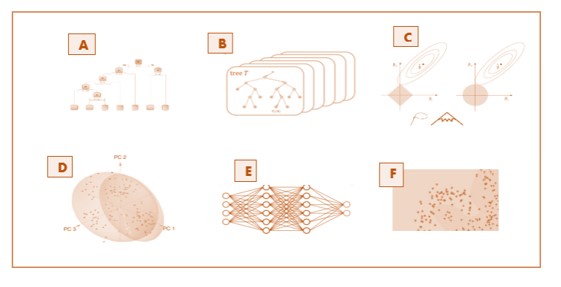
Machine Learning
The application of of machine learning algorithms to clinical trial data is an evolving area, and we are on the forefront. We support these powerful tools with a variety of models such as random forests, support vector machines and neural networks.
Presentation of Results
PowerPoint presentations of the clinical trial outcomes are designed by Integristat in a variety of elegant and accessible formats to address a range of audiences, including academics, researchers, statisticians and regulatory. We have extensive experience with presentations in a large variety of areas

There are undeniable practical advantages to machine learning. The sheer volume and variety of data being generated as humans and other environmental forces interact with technology would be impossible to process and draw insights from without the speed and sophistication of machine learning. From wearable devices that track health and fitness goals or behavior tracer to the chemical and physiological signals - Integristat employs a suite of algorithms leading to a successful data labeling and classification.
Data Preparation
The first step is to get familiar with the data and to clarify the analysis goals. Integristat constructs a robust pipeline to extract the data, automatically clean it and properly characterize it. Dictionaries containing the data labels, types and scales are developed.
Graphical Exploration
Graphical evaluation of the data interplay on a multidimensional figure helps to understand the story. A strong emphasis is placed on visualizing the data on correct and relevant scales in order to draw more insights. Often an effect is demonstrated only on a subset of the data to avoid excess clutter. Some graphical techniques include scaling and the use of multiple aesthetics such as X-Y-Z axes in addition to facets, colors, size and shapes.
Algorithm selection
A Machine learning algorithm is fitted to the data. It is selected from a suite of established models to best suit the learning aim. Some of our popular algorithms include Random Forest, Logistic Regression, GLM, neural networks and SVM. The model is developed on the training subset and again tested on an independent test set.
Algorithm Adjustments
Off-the-self algorithms often provide the first line of prediction if data was properly prepared. Additional custom model adjustments that we apply can improve the algorithm fit to the data and hence considerably improve the classification ability and in turn yield a higher correct classification rate.

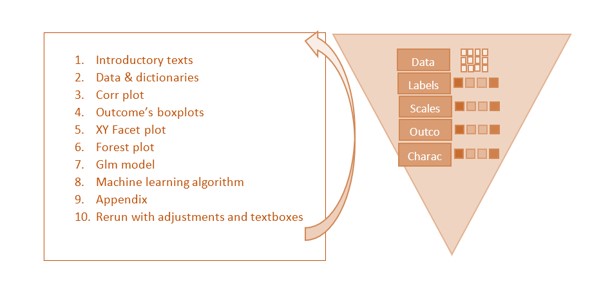
Our automated reporting tool functions as an observational data analysis calculator. Using just 10 steps, it provides a complete report to address the analysis goals, including the tables and graphics required for publication. This is a straightforward tool to quickly extract understanding from any given observational data, speeding up the cycle from data to publication.